- FOR US HEALTHCARE PROFESSIONALS
- FOR PATIENTS & CAREGIVERS»
- Important Safety Information
- Medical Resources
Please see the serious Warnings and Precautions associated with IMFINZI and IMJUDO.
ADVERSE REACTIONS OCCURRING IN ≥10% OF PATIENTS
TREATED WITH IMFINZI + IMJUDO IN THE HIMALAYA STUDY1,2
| All grades (%) | Grades 3-4 (%) | |||
|---|---|---|---|---|
| Adverse reaction | IMFINZI + IMJUDO (n=388) |
Sorafenib (n=374) |
IMFINZI + IMJUDO (n=388) |
Sorafenib (n=374) |
| Skin and subcutaneous tissue disorders | ||||
| Rash* | 32 | 57 | 2.8 | 12 |
| Pruritus | 23 | 6 | 0 | 0.3 |
| Gastrointestinal disorders | ||||
| Diarrhea* | 27 | 45 | 6 | 4.3 |
| Abdominal pain* | 20 | 24 | 1.8 | 4 |
| Nausea | 12 | 14 | 0 | 0 |
| General disorders and administration site conditions | ||||
| Fatigue* | 26 | 30 | 3.9 | 6 |
| Pyrexia* | 13 | 9 | 0.3 | 0.3 |
| Musculoskeletal and connective tissue disorders | ||||
| Musculoskeletal pain* | 22 | 17 | 2.6 | 0.8 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 17 | 18 | 1.3 | 0.8 |
| Endocrine disorders | ||||
| Hypothyroidism* | 14 | 6 | 0 | 0 |
| Psychiatric disorders | ||||
| Insomnia | 10 | 4.3 | 0.3 | 0 |
SCROLL
*Represents a composite of multiple related terms.
Grade 3 or 4 treatment-related AEs occurred in 26% of patients treated with IMFINZI + IMJUDO and 37% of patients treated with sorafenib4
HEMORRHAGE SMQ TREATMENT-RELATED ADVERSE EVENTS REPORTED IN >1 PATIENT IN THE SAFETY ANALYSIS POPULATION OF THE HIMALAYA STUDY4
| Treatment-related AE, n (%) | ||
|---|---|---|
| Adverse event | IMFINZI + IMJUDO (n=388) |
Sorafenib (n=374) |
| Any hemorrhage SMQ event | 7 (1.8) | 18 (4.8) |
| Grade 3 or 4 hemorrhage SMQ event | 2 (0.5) | 4 (1.1) |
| INR increased | 4 (1.0) | 0 |
| Purpura | 2 (0.5) | 1 (0.3) |
| Activated partial thromboplastin time prolonged |
1 (0.3) | 0 |
| Tumor hemorrhage | 1 (0.3) | 0 |
| Epistaxis | 0 | 4 (1.1) |
| Gastrointestinal hemorrhage | 0 | 3 (0.8) |
| Gingival bleeding | 0 | 2 (0.5) |
| Hematuria | 0 | 2 (0.5) |
| Hemoptysis | 0 | 1 (0.3) |
| Hemoglobin decreased | 0 | 1 (0.3) |
| Gastrointestinal ulcer hemorrhage | 0 | 0 |
| Gastric varices hemorrhage | 0 | 0 |
| Hematemesis | 0 | 0 |
| Hematochezia | 0 | 0 |
| Hemorrhage intracranial | 0 | 0 |
| Melena | 0 | 0 |
| Esophageal varices hemorrhage | 0 | 0 |
| Rectal hemorrhage | 0 | 0 |
| Upper gastrointestinal hemorrhage | 0 | 0 |
| Bleeding varicose vein | 0 | 0 |
SCROLL
In the HIMALAYA study, no patients in the IMFINZI + IMJUDO arm experienced treatment-related hemorrhage of gastroesophageal varices4
An exploratory analysis evaluated Child-Pugh A scores and ALBI grades at baseline and over time for IMFINZI + IMJUDO and sorafenib in the HIMALAYA study; not powered for statistical significance10
MEAN CHILD-PUGH SCORES OVER TIME10
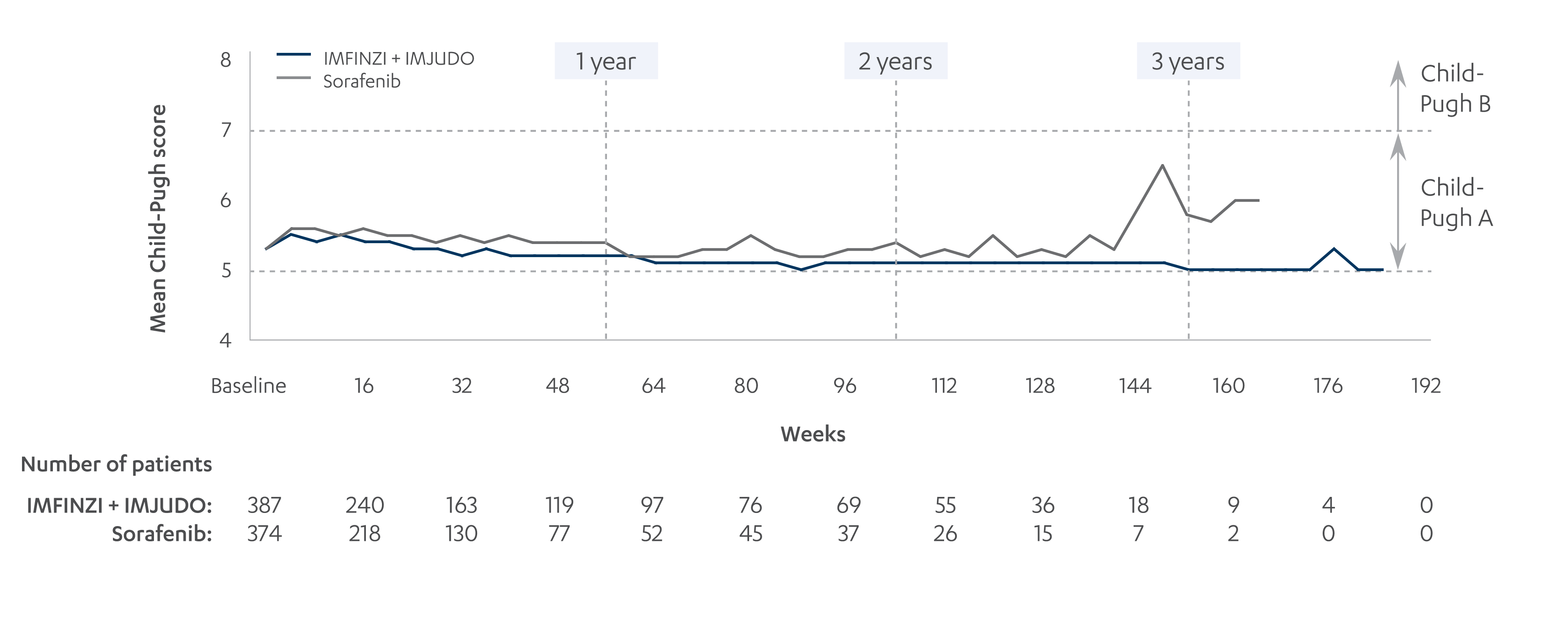
Adapted from Vogel A, et al. 2022.
MEAN ALBI GRADES OVER TIME10
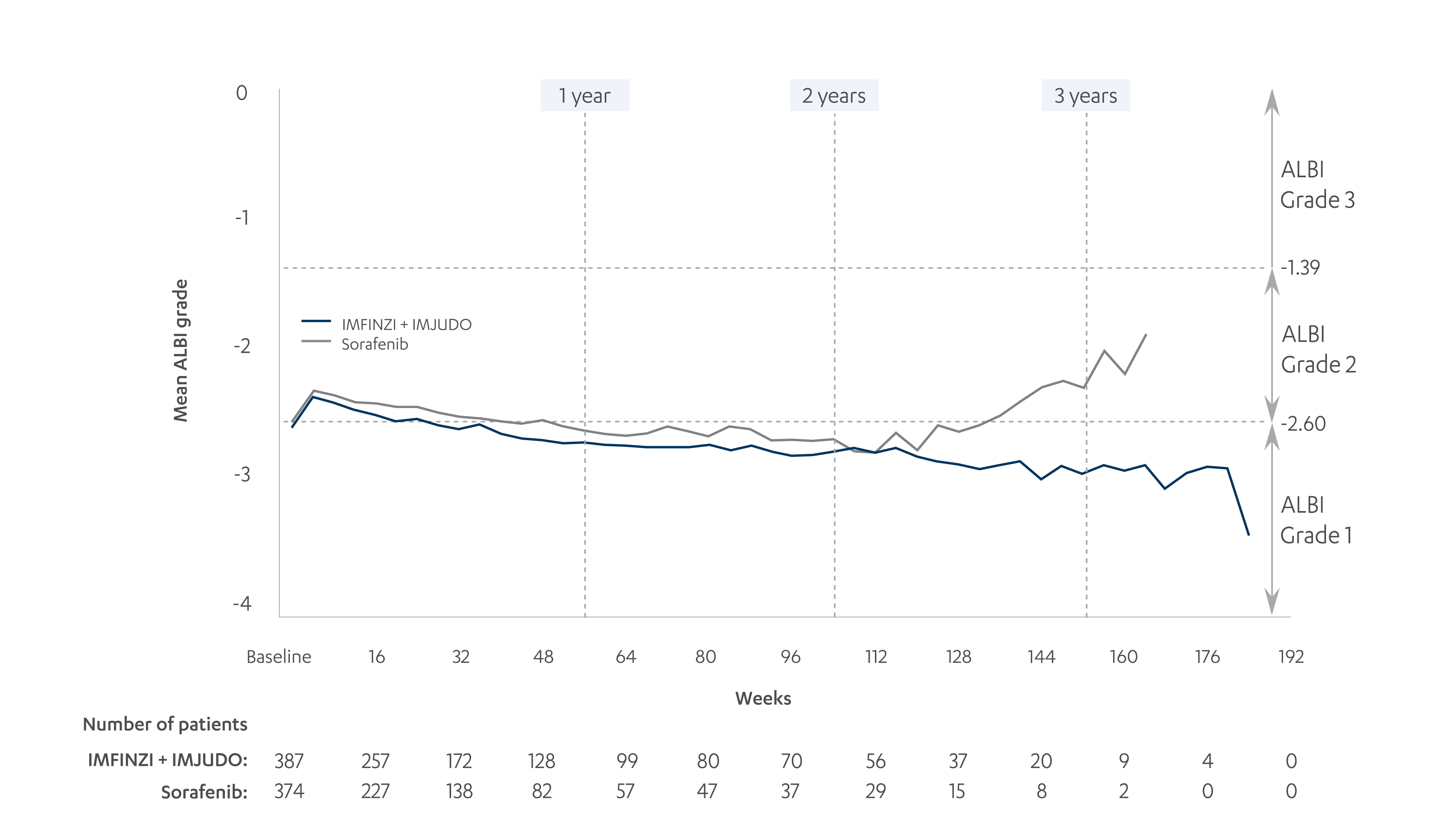
Adapted from Vogel A, et al. 2022.
*Values based on mean Child-Pugh scores and ALBI grades at weekly analysis visits over 3.5 years of treatment. ALBI grade calculated as: log10(bilirubin) × 0.66 − albumin × 0.085.10
IMMUNE-MEDIATED ADVERSE REACTIONS OBSERVED
WITH IMFINZI + IMJUDO IN THE HIMALAYA STUDY4,11
| IMFINZI + IMJUDO (n=388) | |||
|---|---|---|---|
| imARs | All grades (%) | Grades 3-4 (%) | Patients discontinuing due to imARs (%) |
| Any | 35.8 | 12.6 | 5.7 |
| Hepatic events | 7.5 | 4.1 | 2.3 |
| Diarrhea/colitis* | 5.9 | 3.6 | 1.3 |
| Dermatitis/rash† | 4.9 | 1.8 | 0.5 |
| Pancreatic events | 2.3 | 1.8 | 0 |
| Renal events | 1 | 0.5 | 0.5 |
| Adrenal insufficiency | 1.5 | 0.3 | 0 |
| Hyperthyroid events | 4.6 | 0.3 | 0 |
| Hypothyroid events | 10.8 | 0 | 0 |
| Pneumonitis | 1.3 | 0 | 0.3 |
SCROLL
Includes adverse events with onset or increase in severity on or after the date of first dose through 90 days following the date of the last dose or the date of initiation of the first subsequent therapy. Patients may have had >1 event. Events include those that occurred in ≥1% of patients in either treatment arm.
*Immune-mediated diarrhea/colitis was reported as a grouped term within the HIMALAYA study.
†Immune-mediated dermatitis/rash was reported as a grouped term within the HIMALAYA study.
The majority of imARs with IMFINZI + IMJUDO were Grade 1 or 24,12
For patients who experienced an imAR with IMFINZI + IMJUDO, most events occurred within the first 3 months of treatment12
OVERALL FREQUENCY OF ANY imAR BY TIME IN PATIENTS WITH AN imAR (n/N=139/388)12‡
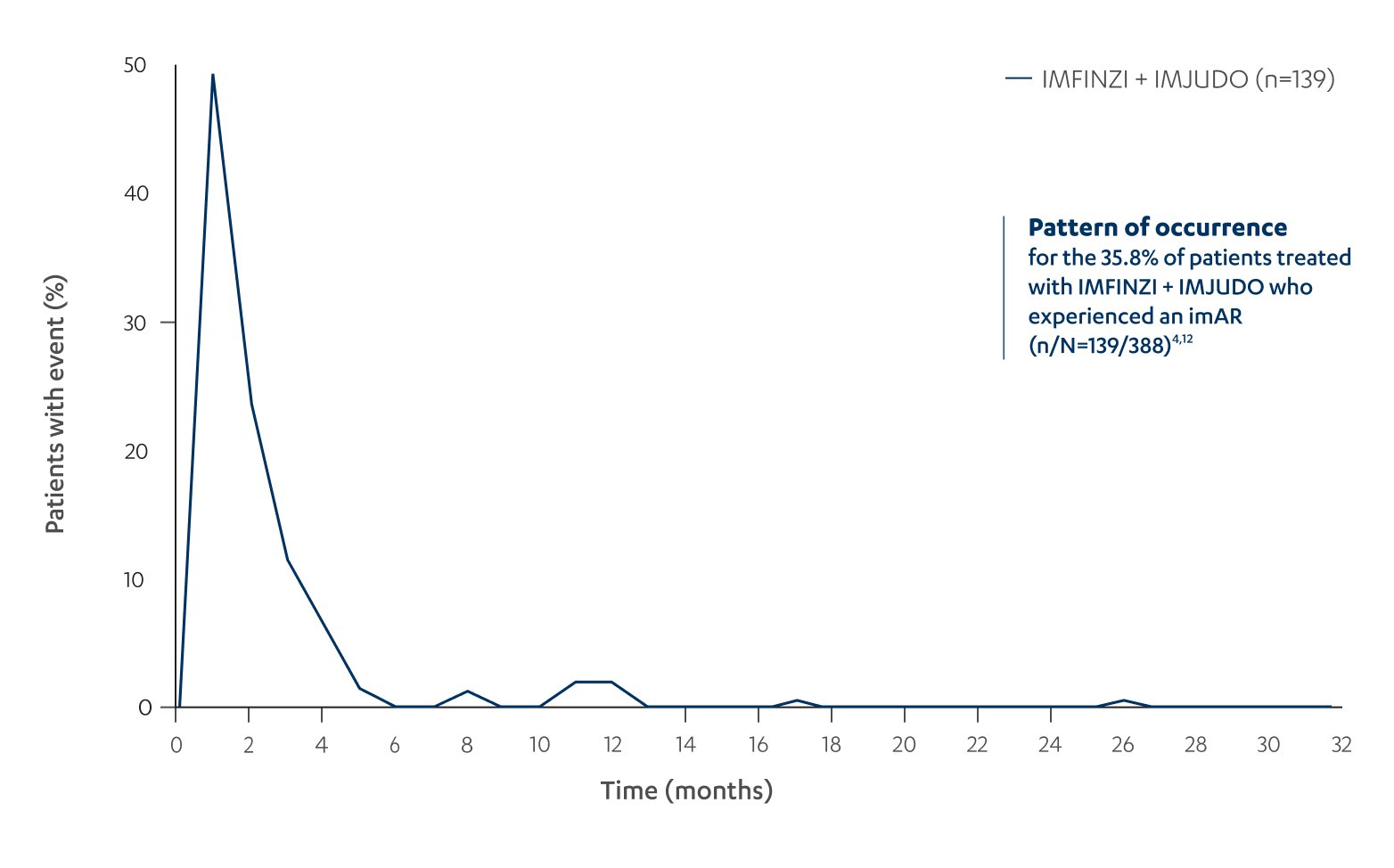
‡The percentage of patients with a reaction is the number of patients who experienced ≥1 imAR at each time interval divided by the number of patients who experienced ≥1 imAR at any time; includes first imARs only, regardless of grade.
imARs, which may be severe or fatal, can occur in any organ system or tissue. imARs can occur at any time after starting treatment or after discontinuation1,2
MOST COMMON imARS BY TIME IN PATIENTS WITH AN imAR12§
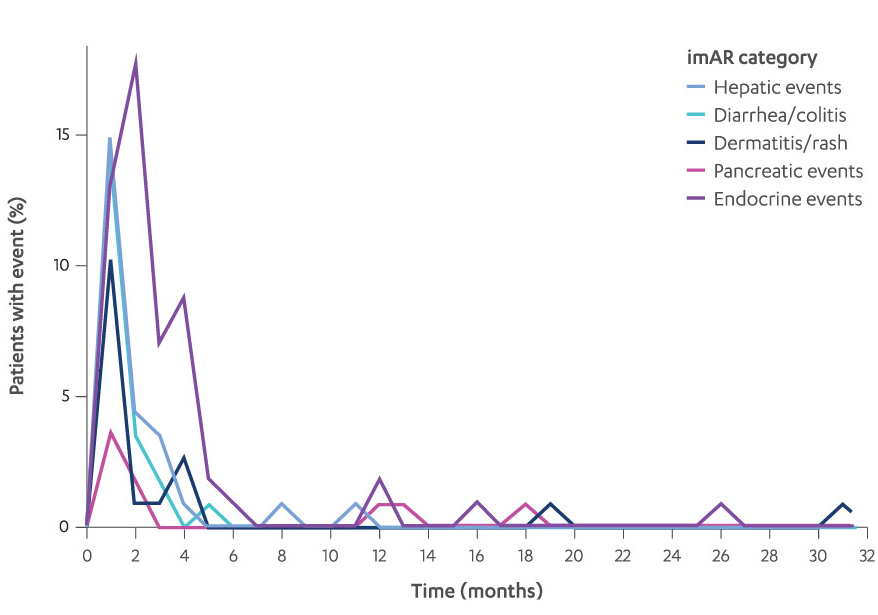
§The percentage of patients with a reaction is the number of patients who experienced ≥1 imAR at each time interval divided by the number of patients who experienced ≥1 imAR at any time; includes the first imARs only, regardless of grade.
LAB ABNORMALITIES WORSENING FROM BASELINE OCCURRING IN ≥20% OF PATIENTS TREATED WITH IMFINZI + IMJUDO IN THE HIMALAYA STUDY1,2
| Any grade* (%)† | Grades 3*-4 (%)† | |||
|---|---|---|---|---|
| Lab abnormality | IMFINZI + IMJUDO |
Sorafenib |
IMFINZI + IMJUDO |
Sorafenib |
| Chemistry | ||||
| Aspartate aminotransferase increased |
63 | 55 | 27 | 21 |
| Alanine aminotransferase increased |
56 | 53 | 18 | 12 |
| Sodium decreased | 46 | 40 | 15 | 11 |
| Bilirubin increased | 41 | 47 | 8 | 11 |
| Alkaline phosphatase increased |
41 | 44 | 8 | 5 |
| Glucose increased | 39 | 29 | 14 | 4 |
| Calcium decreased | 34 | 43 | 0 | 0.3 |
| Albumin decreased | 31 | 37 | 0.5 | 1.7 |
| Potassium increased | 28 | 21 | 3.8 | 2.6 |
| Creatinine increased | 21 | 15 | 1.3 | 0.9 |
| Hematology | ||||
| Hemoglobin decreased | 52 | 40 | 4.8 | 6 |
| Lymphocytes decreased | 41 | 39 | 11 | 10 |
| Platelets decreased | 29 | 35 | 1.6 | 3.1 |
| Leukocytes decreased | 20 | 30 | 0.8 | 1.1 |
SCROLL
*Graded according to NCI CTCAE version 4.03.
†Each test incidence is based on the number of patients who had both baseline and at least 1 on-study laboratory measurement available: IMFINZI + IMJUDO (range: 367-378) and sorafenib (range: 344-352).


IMFINZI is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
There are no contraindications for IMFINZI® (durvalumab) or IMJUDO® (tremelimumab-actl).
Severe and Fatal Immune-Mediated
Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and before each dose. In cases of suspected immune-mediated adverse reactions, initiate
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
There are no contraindications for IMFINZI® (durvalumab) or IMJUDO® (tremelimumab-actl).
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and before each dose. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI and IMJUDO depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI and IMJUDO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle-invasive bladder cancer (MIBC).
IMFINZI in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) as neoadjuvant and adjuvant treatment, followed by single agent IMFINZI, is indicated for the treatment of adult patients with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
IMFINZI and IMJUDO can cause immune-mediated pneumonitis, which may be fatal. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
IMFINZI with IMJUDO and platinum-based chemotherapy can cause immune-mediated colitis, which may be
fatal.
IMFINZI and IMJUDO can cause immune-mediated colitis that is frequently associated with diarrhea.
Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory
immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup
to exclude alternative etiologies.
IMFINZI and IMJUDO can cause immune-mediated hepatitis, which may be fatal.
IMFINZI and IMJUDO can cause immune-mediated nephritis.
IMFINZI and IMJUDO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 and CTLA-4 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.
IMFINZI in combination with IMJUDO can cause immune-mediated pancreatitis. Immune-mediated pancreatitis occurred in 2.3% (9/388) of patients receiving IMFINZI and IMJUDO, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions.
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI and IMJUDO or were reported with the use of other immune-checkpoint inhibitors.
IMFINZI and IMJUDO can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI and IMJUDO based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Based on their mechanism of action and data from animal studies, IMFINZI and IMJUDO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and IMJUDO and advise them to use effective contraception during treatment with IMFINZI and IMJUDO and for 3 months after the last dose of IMFINZI and IMJUDO.
There is no information regarding the presence of IMFINZI and IMJUDO in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI and IMJUDO, advise women not to breastfeed during treatment and for 3 months after the last dose.
The safety and effectiveness of IMFINZI and IMJUDO have not been established in pediatric patients.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle-invasive bladder cancer (MIBC).
IMFINZI in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) as neoadjuvant and adjuvant treatment, followed by single agent IMFINZI, is indicated for the treatment of adult patients with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
Please see Full Prescribing Information including Medication Guide for IMFINZI and IMJUDO.
You may report side effects
related to AstraZeneca products  .
.