IMPORTANT SAFETY INFORMATION
There are no contraindications for IMFINZI® (durvalumab) or
IMJUDO® (tremelimumab-actl).
Severe and Fatal Immune-Mediated Adverse Reactions
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all
possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in
any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or
after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of
underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine,
adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and before each dose. In cases of
suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies,
including infection. Institute medical management promptly, including specialty consultation as appropriate.
Withhold or permanently discontinue IMFINZI and IMJUDO depending on severity. See USPI Dosing and Administration
for specific details. In general, if IMFINZI and IMJUDO requires interruption or discontinuation, administer systemic
corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon
improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider
administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not
controlled with corticosteroid therapy.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage
III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based
chemotherapy and radiation therapy (cCRT).
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI
continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients
with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR)
mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult
patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer
(LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation
therapy (cCRT).
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line
treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients
with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable
hepatocellular carcinoma (uHCC).
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle-invasive bladder cancer (MIBC).
IMFINZI in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) as neoadjuvant and adjuvant treatment, followed by single agent IMFINZI, is indicated for the treatment of adult patients with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
Immune-Mediated Pneumonitis
IMFINZI and IMJUDO can cause immune-mediated pneumonitis, which may be fatal. The
incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
- IMFINZI as a Single Agent
-
- In patients who did not receive recent prior radiation, the incidence of immune-mediated pneumonitis was 2.4% (34/1414), including fatal (<0.1%), and Grade 3-4 (0.4%) adverse reactions.
- In patients who received recent prior radiation, the incidence of pneumonitis (including radiation pneumonitis) in patients with unresectable Stage III NSCLC following definitive chemoradiation within 42 days prior to initiation of IMFINZI in PACIFIC was 18.3% (87/475) in patients receiving IMFINZI and 12.8% (30/234) in patients receiving placebo. Of the patients who received IMFINZI (475), 1.1% were fatal and 2.7% were Grade 3 adverse reactions.
- The incidence of pneumonitis (including radiation pneumonitis) in patients with LS-SCLC following chemoradiation within 42 days prior to initiation of IMFINZI in ADRIATIC was 14% (37/262) in patients receiving IMFINZI and 6% (16/265) in patients receiving placebo. Of the patients who received IMFINZI (262), 0.4% had a fatal adverse reaction and 2.7% had Grade 3 adverse reactions.
- The frequency and severity of immune-mediated pneumonitis in patients who did not receive definitive chemoradiation prior to IMFINZI were similar in patients who received IMFINZI as a single agent or with ES-SCLC or BTC when given in combination with chemotherapy.
- IMFINZI with IMJUDO
-
- Immune-mediated pneumonitis occurred in 1.3% (5/388) of patients receiving IMFINZI and IMJUDO, including fatal (0.3%) and Grade 3 (0.2%) adverse reactions.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated pneumonitis occurred in 3.5% (21/596) of patients receiving IMFINZI in
combination with IMJUDO and platinum-based chemotherapy,
including fatal (0.5%), and Grade 3 (1%) adverse reactions.
Immune-Mediated Colitis
IMFINZI with IMJUDO and platinum-based chemotherapy can cause immune-mediated colitis, which may be
fatal.
IMFINZI and IMJUDO can cause immune-mediated colitis that is frequently associated with diarrhea.
Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory
immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup
to exclude alternative etiologies.
- IMFINZI as a Single Agent
-
- Immune-mediated colitis occurred in 2% (37/1889) of patients receiving IMFINZI, including Grade 4
(<0.1%) and Grade 3 (0.4%) adverse reactions.
- IMFINZI with IMJUDO
-
- Immune-mediated colitis or diarrhea occurred in 6% (23/388) of patients receiving IMFINZI and
IMJUDO, including Grade 3 (3.6%) adverse reactions. Intestinal perforation has been observed in other
studies of IMFINZI and IMJUDO.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated colitis occurred in 6.5% (39/596) of patients receiving IMFINZI in
combination with IMJUDO and platinum-based chemotherapy including fatal (0.2%) and Grade 3
(2.5%) adverse reactions. Intestinal perforation and large intestine perforation were reported
in 0.1% of patients.
Immune-Mediated Hepatitis
IMFINZI and IMJUDO can cause immune-mediated hepatitis, which may be fatal.
- IMFINZI as a Single Agent
-
- Immune-mediated hepatitis occurred in 2.8% (52/1889) of patients receiving IMFINZI, including fatal
(0.2%), Grade 4 (0.3%) and Grade 3 (1.4%) adverse reactions.
- IMFINZI with IMJUDO
-
- Immune-mediated hepatitis occurred in 7.5% (29/388) of patients receiving IMFINZI and IMJUDO,
including fatal (0.8%), Grade 4 (0.3%) and Grade 3 (4.1%) adverse reactions.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated hepatitis occurred in 3.9% (23/596) of patients receiving IMFINZI in
combination with IMJUDO and platinum-based chemotherapy, including fatal (0.3%), Grade 4 (0.5%),
and Grade 3 (2%) adverse reactions.
Immune-Mediated Endocrinopathies
- Adrenal Insufficiency: IMFINZI and IMJUDO can cause primary
or secondary adrenal insufficiency. For Grade 2 or higher adrenal insufficiency, initiate symptomatic treatment,
including hormone replacement as clinically indicated.
-
- IMFINZI as a Single Agent
-
- Immune-mediated adrenal insufficiency occurred in 0.5% (9/1889) of
patients receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
- IMFINZI with IMJUDO
-
- Immune-mediated adrenal insufficiency occurred in 1.5% (6/388) of patients
receiving IMFINZI and IMJUDO, including Grade 3 (0.3%) adverse reactions.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated adrenal insufficiency occurred in 2.2% (13/596) of
patients receiving IMFINZI in combination with IMJUDO and platinum-based chemotherapy, including
Grade 3 (0.8%) adverse reactions.
- Hypophysitis: IMFINZI and IMJUDO can cause immune-mediated hypophysitis.
Hypophysitis can present with acute symptoms associated with mass effect such as headache, photophobia, or visual
field cuts. Hypophysitis can cause hypopituitarism. Initiate symptomatic treatment including hormone replacement
as clinically indicated.
-
- IMFINZI as a Single Agent
-
- Grade 3 hypophysitis/hypopituitarism occurred in <0.1% (1/1889) of
patients who received IMFINZI.
- IMFINZI with IMJUDO
-
- Immune-mediated hypophysitis/hypopituitarism occurred in 1% (4/388) of
patients receiving IMFINZI and IMJUDO.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated hypophysitis occurred in 1.3% (8/596) of patients
receiving IMFINZI in combination with IMJUDO and platinum-based chemotherapy, including Grade 3
(0.5%) adverse reactions.
- Thyroid Disorders (Thyroiditis, Hyperthyroidism, and
Hypothyroidism): IMFINZI and IMJUDO can cause immune-mediated thyroid disorders. Thyroiditis can
present with or without endocrinopathy. Hypothyroidism can follow hyperthyroidism. Initiate hormone replacement
therapy for hypothyroidism or institute medical management of hyperthyroidism as clinically indicated.
-
- IMFINZI as a Single Agent
-
- Immune-mediated thyroiditis occurred in 0.5% (9/1889) of patients
receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
- Immune-mediated hyperthyroidism occurred in 2.1% (39/1889) of patients
receiving IMFINZI.
- Immune-mediated hypothyroidism occurred in 8.3% (156/1889) of patients
receiving IMFINZI, including Grade 3 (<0.1%) adverse reactions.
- IMFINZI with IMJUDO
-
- Immune-mediated thyroiditis occurred in 1.5% (6/388) of patients receiving IMFINZI and IMJUDO.
- Immune-mediated hyperthyroidism occurred in 4.6% (18/388) of patients receiving IMFINZI and IMJUDO, including Grade 3 (0.3%) adverse reactions.
- Immune-mediated hypothyroidism occurred in 11% (42/388) of patients receiving IMFINZI and IMJUDO.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated thyroiditis occurred in 1.2% (7/596) of patients receiving
IMFINZI in combination with IMJUDO and platinum-based chemotherapy.
- Immune-mediated hyperthyroidism occurred in 5% (30/596) of patients
receiving IMFINZI in combination with IMJUDO and platinum-based chemotherapy, including Grade 3 (0.2%) adverse reactions.
- Immune-mediated hypothyroidism occurred in 8.6% (51/596) of patients receiving
IMFINZI in combination with IMJUDO and platinum-based chemotherapy, including Grade 3 (0.5%) adverse
reactions.
- IMFINZI with Carboplatin and Paclitaxel
-
- Immune-mediated hypothyroidism occurred in 14% (34/235) of patients
receiving IMFINZI in combination with carboplatin and paclitaxel.
- Type 1 Diabetes Mellitus, which can present with diabetic ketoacidosis:
Monitor patients for hyperglycemia or other signs and symptoms of diabetes. Initiate treatment with insulin as clinically indicated.
-
- IMFINZI as a Single Agent
-
- Grade 3 immune-mediated Type 1 diabetes mellitus occurred in <0.1%
(1/1889) of patients receiving IMFINZI.
- IMFINZI with IMJUDO
-
- Two patients (0.5%, 2/388) had events of hyperglycemia requiring insulin
therapy that had not resolved at last follow-up.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated Type 1 diabetes mellitus occurred in 0.5% (3/596) of
patients receiving IMFINZI in combination with IMJUDO and platinum-based chemotherapy including Grade 3 (0.3%) adverse
reactions.
Immune-Mediated Nephritis with Renal Dysfunction
IMFINZI and IMJUDO can cause immune-mediated nephritis.
- IMFINZI as a Single Agent
-
- Immune-mediated nephritis occurred in 0.5% (10/1889) of patients receiving IMFINZI, including Grade 3
(<0.1%) adverse reactions.
- IMFINZI with IMJUDO
-
- Immune-mediated nephritis occurred in 1% (4/388) of patients receiving IMFINZI and IMJUDO, including
Grade 3 (0.5%) adverse reactions.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated nephritis occurred in 0.7% (4/596) of patients receiving IMFINZI in combination with
IMJUDO and platinum-based chemotherapy, including Grade 3 (0.2%) adverse reactions.
Immune-Mediated Dermatology Reactions
IMFINZI and IMJUDO can cause immune-mediated rash or dermatitis. Exfoliative
dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and
toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 and CTLA-4 blocking antibodies. Topical emollients and/or topical corticosteroids may be
adequate to treat mild to moderate non-exfoliative rashes.
- IMFINZI as a Single Agent
-
- Immune-mediated rash or dermatitis occurred in 1.8% (34/1889) of patients receiving IMFINZI, including
Grade 3 (0.4%) adverse reactions.
- IMFINZI with IMJUDO
-
- Immune-mediated rash or dermatitis occurred in 4.9% (19/388) of patients receiving IMFINZI and IMJUDO,
including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Immune-mediated rash or dermatitis occurred in 7.2% (43/596) of patients receiving IMFINZI in
combination with IMJUDO and platinum-based chemotherapy, including Grade 3 (0.3%) adverse reactions.
Immune-Mediated Pancreatitis
IMFINZI in combination with IMJUDO can cause immune-mediated pancreatitis. Immune-mediated pancreatitis occurred in 2.3% (9/388) of patients receiving IMFINZI
and IMJUDO, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions.
Other Immune-Mediated Adverse Reactions
The following clinically significant, immune-mediated adverse reactions occurred at
an incidence of less than 1% each in patients who received IMFINZI and IMJUDO or were reported with the use of other
immune-checkpoint inhibitors.
- Cardiac/vascular: Myocarditis, pericarditis, vasculitis.
- Nervous system: Meningitis, encephalitis, myelitis and demyelination,
myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré syndrome, nerve paresis,
autoimmune neuropathy.
- Ocular: Uveitis, iritis, and other ocular inflammatory toxicities can occur.
Some cases can be associated with retinal detachment. Various grades of visual impairment to include blindness can occur.
If uveitis occurs in combination with other immune-mediated adverse reactions, consider a Vogt-Koyanagi-Harada-like syndrome,
as this may require treatment with systemic steroids to reduce the risk of permanent vision loss.
- Gastrointestinal: Pancreatitis including increases in serum amylase and
lipase levels, gastritis, duodenitis.
- Musculoskeletal and connective tissue disorders: Myositis/polymyositis,
rhabdomyolysis and associated sequelae including renal failure, arthritis, polymyalgia rheumatic.
- Endocrine: Hypoparathyroidism.
- Other (hematologic/immune): Hemolytic anemia, aplastic anemia, hemophagocytic
lymphohistiocytosis, systemic inflammatory response syndrome, histiocytic necrotizing lymphadenitis (Kikuchi lymphadenitis),
sarcoidosis, immune thrombocytopenia, solid organ transplant rejection, other transplant (including corneal graft) rejection.
Infusion-Related Reactions
IMFINZI and IMJUDO can cause severe or life-threatening infusion-related reactions.
Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the
rate of, or permanently discontinue IMFINZI and IMJUDO based on the severity. See USPI Dosing and Administration for
specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.
- IMFINZI as a Single Agent
-
- Infusion-related reactions occurred in 2.2% (42/1889) of patients receiving IMFINZI, including Grade 3
(0.3%) adverse reactions.
- IMFINZI with IMJUDO
-
- Infusion-related reactions occurred in 2.6% (10/388) of patients receiving IMFINZI and IMJUDO.
- IMFINZI with IMJUDO and Platinum-Based Chemotherapy
-
- Infusion-related reactions occurred in 2.9% (17/596) of patients receiving IMFINZI in combination with
IMJUDO and platinum-based chemotherapy, including Grade 3 (0.3%) adverse reactions.
Complications of Allogeneic HSCT after IMFINZI
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell
transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related
complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive
disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified
infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT.
Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus
risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Embryo-Fetal Toxicity
Based on their mechanism of action and data from animal studies, IMFINZI and IMJUDO can cause fetal harm when
administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive
potential, verify pregnancy status prior to initiating IMFINZI and IMJUDO and advise them to use effective contraception
during treatment with IMFINZI and IMJUDO and for 3 months after the last dose of IMFINZI and IMJUDO.
Lactation
There is no information regarding the presence of IMFINZI and IMJUDO in human milk; however, because of the potential
for serious adverse reactions in breastfed infants from IMFINZI and IMJUDO, advise women not to breastfeed during treatment
and for 3 months after the last dose.
Adverse Reactions
Unresectable Stage III NSCLC
- In patients with Stage III NSCLC in the PACIFIC study receiving IMFINZI (n=475), the most common adverse
reactions (≥20%) were cough (40%), fatigue (34%), pneumonitis or radiation pneumonitis (34%), upper respiratory
tract infections (26%), dyspnea (25%), and rash (23%). The most common Grade 3 or 4 adverse reactions (≥3%) were
pneumonia (7%) and pneumonitis/radiation pneumonitis (3.4%).
- In patients with Stage III NSCLC in the PACIFIC study receiving IMFINZI (n=475), discontinuation due to
adverse reactions occurred in 15% of patients in the IMFINZI arm. Serious adverse reactions occurred in 29% of
patients receiving IMFINZI. The most frequent serious adverse reactions (≥2%) were pneumonitis or radiation pneumonitis
(7%) and pneumonia (6%). Fatal pneumonitis or radiation pneumonitis and fatal pneumonia occurred in <2% of patients and
were similar across arms.
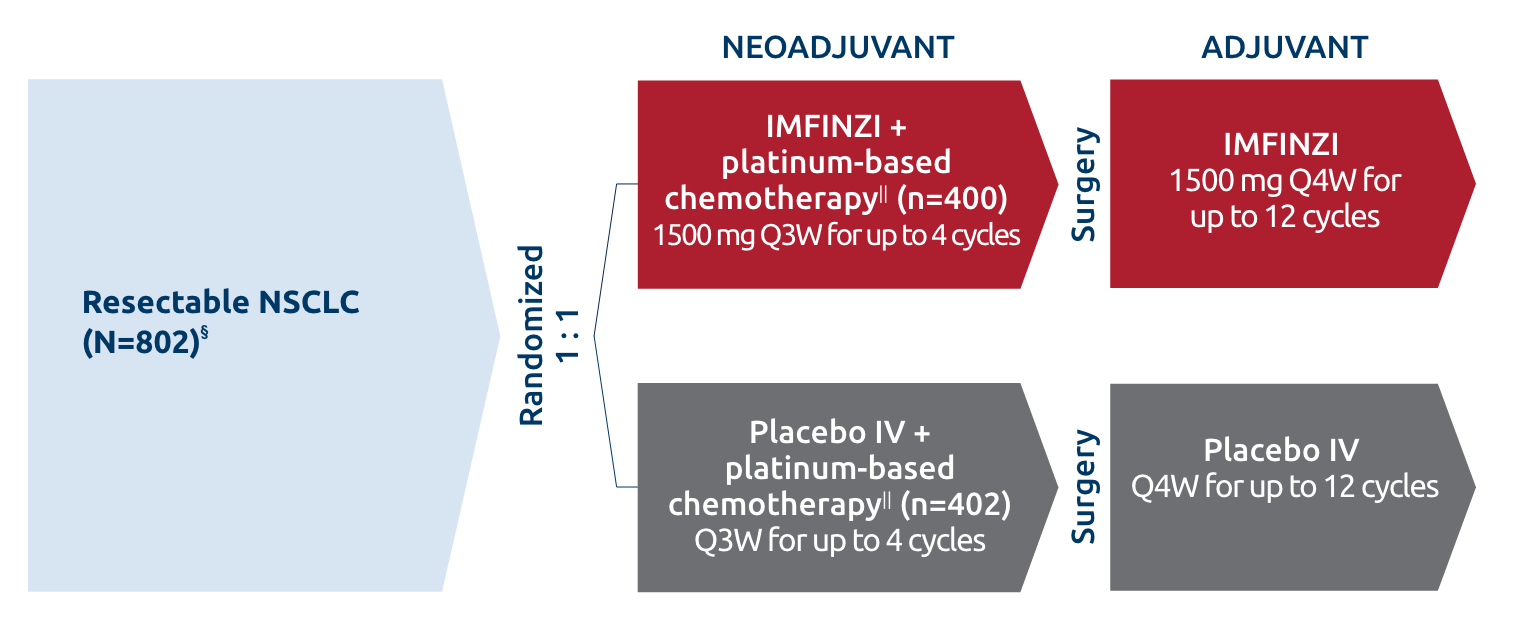
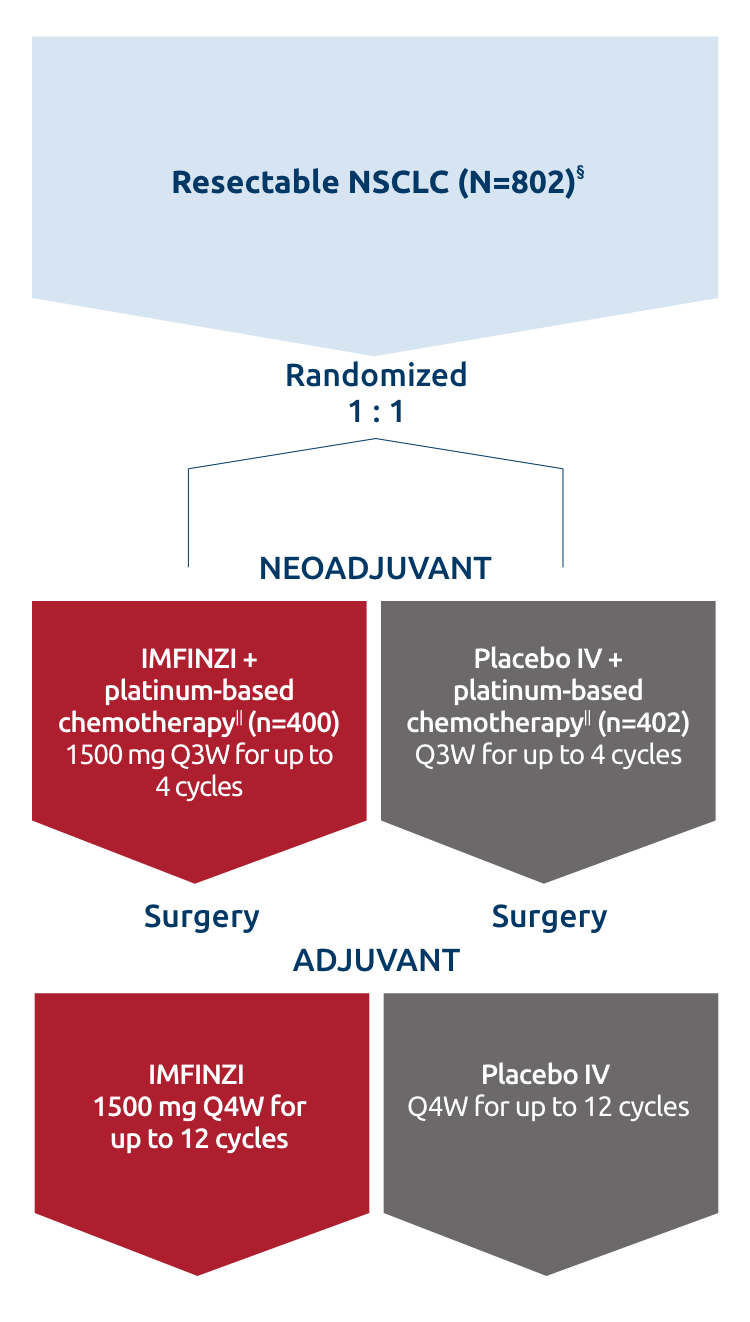
Resectable NSCLC
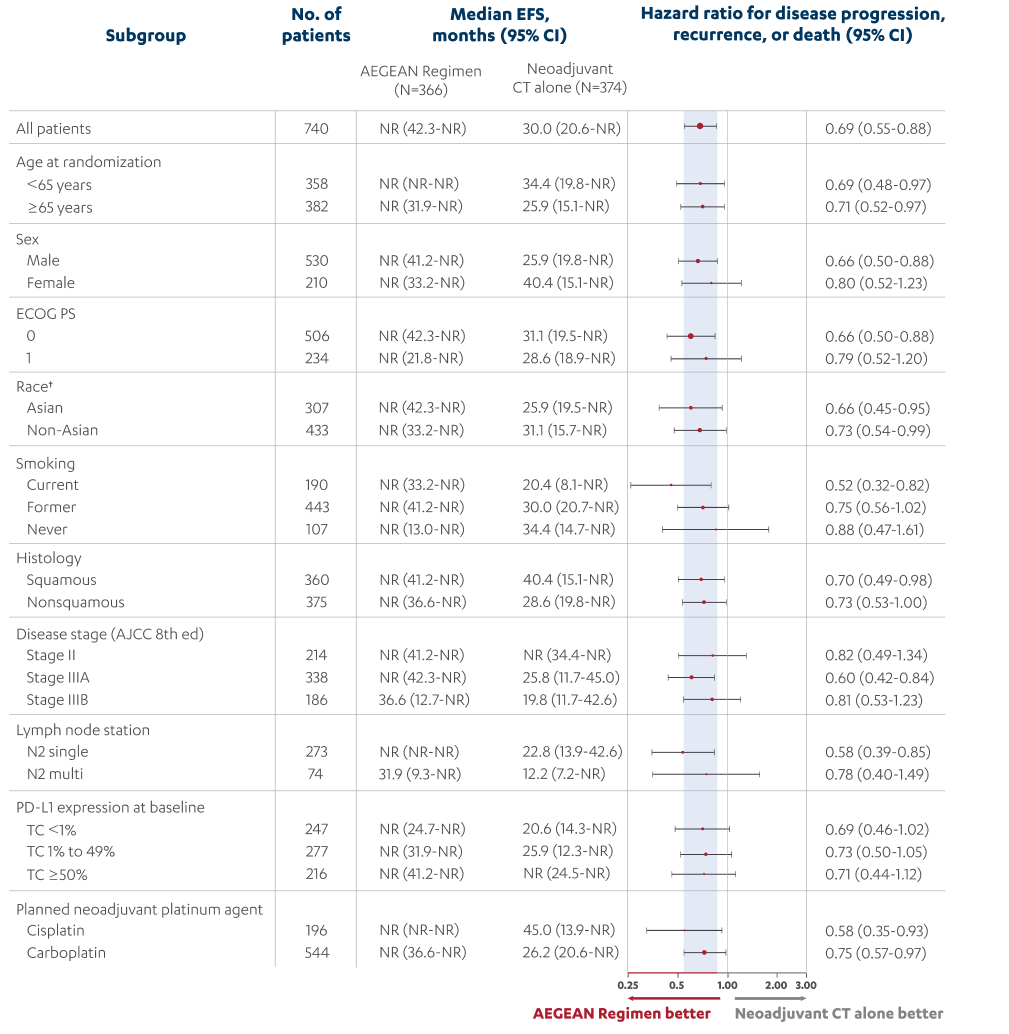
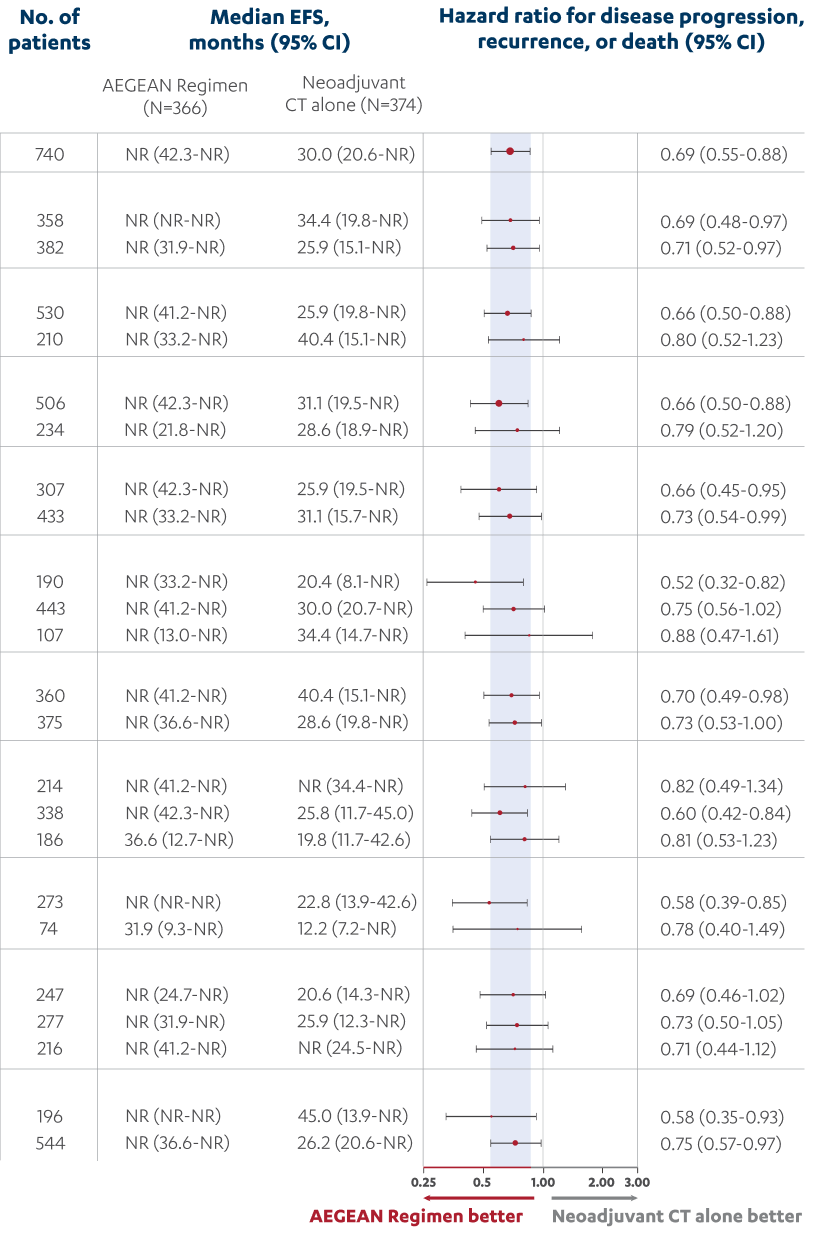
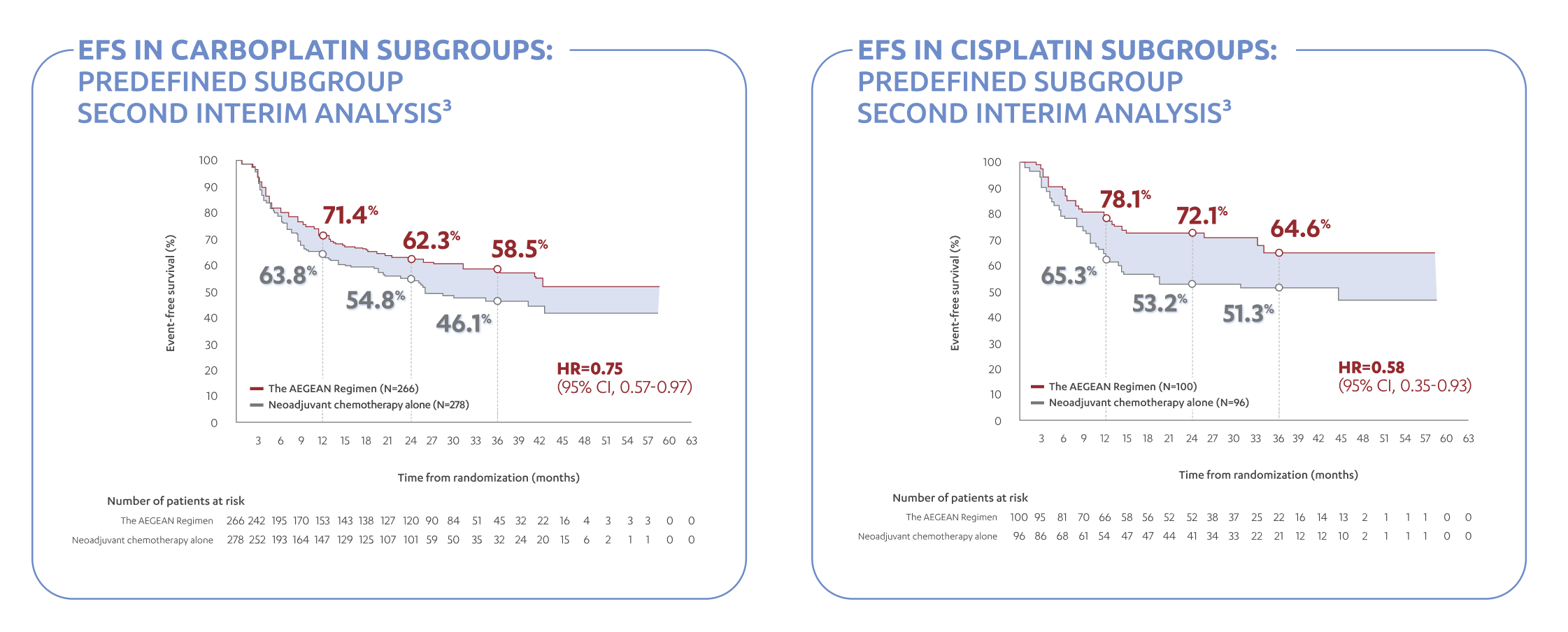
- In patients with resectable NSCLC in the AEGEAN study, the most common adverse reactions (occurring in ≥20% of patients)
were anemia, nausea, constipation, fatigue, musculoskeletal pain, and rash.
- In patients with resectable NSCLC in the neoadjuvant phase of the AEGEAN study receiving IMFINZI in combination with
platinum-containing chemotherapy (n=401), permanent discontinuation of IMFINZI due to an adverse reaction occurred in 6.7% of
patients. Serious adverse reactions occurred in 21% of patients. The most frequent (≥1%) serious adverse reactions were
pneumonia (2.7%), anemia (1.5%), myelosuppression (1.5%), vomiting (1.2%), neutropenia (1%), and acute kidney injury (1%).
Fatal adverse reactions occurred in 2% of patients, including death due to COVID-19 pneumonia (0.5%), sepsis (0.5%),
myocarditis (0.2%), decreased appetite (0.2%), hemoptysis (0.2%), and death not otherwise specified (0.2%). Of the 401
IMFINZI-treated patients who received neoadjuvant treatment and 398 placebo-treated patients who received neoadjuvant
treatment, 1.7% (n=7) and 1% (n=4), respectively, did not receive surgery due to adverse reactions.
- In patients with resectable NSCLC in the adjuvant phase of the AEGEAN study receiving IMFINZI as a single agent
(n=265), permanent discontinuation of IMFINZI due to an adverse reaction occurred in 8% of patients. Serious adverse
reactions occurred in 13% of patients. The most frequent serious adverse reactions reported in >1% of patients were
pneumonia (1.9%), pneumonitis (1.1%), and COVID-19 (1.1%). Four fatal adverse reactions occurred during the adjuvant
phase of the study, including COVID-19 pneumonia, pneumonia aspiration, interstitial lung disease and aortic aneurysm.
Metastatic NSCLC
- In patients with mNSCLC in the POSEIDON study receiving IMFINZI and IMJUDO plus platinum-based chemotherapy
(n=330), the most common adverse reactions (occurring in ≥20% of patients) were nausea (42%), fatigue (36%),
musculoskeletal pain (29%), decreased appetite (28%), rash (27%), and diarrhea (22%).
- In patients with mNSCLC in the POSEIDON study receiving IMFINZI in combination with IMJUDO and
platinum-based chemotherapy (n=330), permanent discontinuation of IMFINZI or IMJUDO due to an adverse
reaction occurred in 17% of patients. Serious adverse reactions occurred in 44% of patients, with the
most frequent serious adverse reactions reported in at least 2% of patients being pneumonia (11%), anemia
(5%), diarrhea (2.4%), thrombocytopenia (2.4%), pyrexia (2.4%), and febrile neutropenia (2.1%). Fatal adverse
reactions occurred in a total of 4.2% of patients.
Limited-stage Small Cell Lung Cancer
- In patients with limited-stage SCLC in the ADRIATIC study receiving IMFINZI (n=262),
the most common adverse reactions occurring in ≥20% of patients receiving IMFINZI were pneumonitis or
radiation pneumonitis (38%), and fatigue (21%). The most common Grade 3 or 4 adverse reactions
(≥3%) were pneumonitis or radiation pneumonitis and pneumonia.
- In patients with limited-stage SCLC in the ADRIATIC study receiving IMFINZI (n=262), IMFINZI was
permanently discontinued due to adverse reactions in 16% of the patients receiving IMFINZI. Serious
adverse reactions occurred in 30% of patients receiving IMFINZI. The most frequent serious adverse
reactions reported in ≥1% of patients receiving IMFINZI were pneumonitis or radiation pneumonitis
(12%), and pneumonia (5%). Fatal adverse reactions occurred in 2.7% of patients who received IMFINZI
including pneumonia (1.5%), cardiac failure, encephalopathy and pneumonitis (0.4% each).
Extensive-stage Small Cell Lung Cancer
- In patients with extensive-stage SCLC in the CASPIAN study receiving IMFINZI plus chemotherapy (n=265),
the most common adverse reactions (≥20%) were nausea (34%), fatigue/asthenia (32%), and alopecia (31%). The most
common Grade 3 or 4 adverse reaction (≥3%) was fatigue/asthenia (3.4%).
- In patients with extensive-stage SCLC in the CASPIAN study receiving IMFINZI plus chemotherapy (n=265),
IMFINZI was discontinued due to adverse reactions in 7% of the patients receiving IMFINZI plus chemotherapy.
Serious adverse reactions occurred in 31% of patients receiving IMFINZI plus chemotherapy. The most frequent
serious adverse reactions reported in at least 1% of patients were febrile neutropenia (4.5%), pneumonia (2.3%),
anemia (1.9%), pancytopenia (1.5%), pneumonitis (1.1%), and COPD (1.1%). Fatal adverse reactions occurred in 4.9%
of patients receiving IMFINZI plus chemotherapy.
Locally Advanced or Metastatic Biliary Tract Cancers
- In patients with locally advanced or metastatic BTC in the TOPAZ-1 study receiving IMFINZI (n=338),
the most common adverse reactions (occurring in ≥20% of patients) were fatigue (42%), nausea (40%), constipation
(32%), decreased appetite (26%), abdominal pain (24%), rash (23%), and pyrexia (20%).
- In patients with locally advanced or metastatic BTC in the TOPAZ-1 study receiving IMFINZI (n=338),
discontinuation due to adverse reactions occurred in 6% of the patients receiving IMFINZI plus chemotherapy.
Serious adverse reactions occurred in 47% of patients receiving IMFINZI plus chemotherapy. The most frequent
serious adverse reactions reported in at least 2% of patients were cholangitis (7%), pyrexia (3.8%), anemia
(3.6%), sepsis (3.3%) and acute kidney injury (2.4%). Fatal adverse reactions occurred in 3.6% of patients
receiving IMFINZI plus chemotherapy. These include ischemic or hemorrhagic stroke (4 patients), sepsis (2 patients),
and upper gastrointestinal hemorrhage (2 patients).
Unresectable Hepatocellular Carcinoma
- In patients with unresectable HCC in the HIMALAYA study receiving IMFINZI and IMJUDO (n=388), the most common
adverse reactions (occurring in ≥20% of patients) were rash (32%), diarrhea (27%), fatigue (26%), pruritus (23%),
musculoskeletal pain (22%), and abdominal pain (20%).
- In patients with unresectable HCC in the HIMALAYA study receiving IMFINZI and IMJUDO (n=388), serious adverse
reactions occurred in 41% of patients. Serious adverse reactions in >1% of patients included hemorrhage (6%), diarrhea
(4%), sepsis (2.1%), pneumonia (2.1%), rash (1.5%), vomiting (1.3%), acute kidney injury (1.3%), and anemia (1.3%).
Fatal adverse reactions occurred in 8% of patients who received IMFINZI and IMJUDO, including death (1%), hemorrhage
intracranial (0.5%), cardiac arrest (0.5%), pneumonitis (0.5%), hepatic failure (0.5%), and immune-mediated hepatitis
(0.5%). Permanent discontinuation of treatment regimen due to an adverse reaction occurred in 14% of patients.
Primary advanced or Recurrent dMMR Endometrial Cancer
- In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination
with carboplatin and paclitaxel followed by IMFINZI as a single agent (n=44), the most common adverse reactions,
including laboratory abnormalities (occurring in >20% of patients) were peripheral neuropathy (61%), musculoskeletal
pain (59%), nausea (59%), alopecia (52%), fatigue (41%), abdominal pain (39%), constipation (39%), rash (39%), decreased
magnesium (36%), increased ALT (32%), increased AST (30%), diarrhea (27%), vomiting (27%), cough (27%), decreased
potassium (25%), dyspnea (25%), headache (23%), increased alkaline phosphatase (20%), and decreased appetite (18%).
The most common Grade 3 or 4 adverse reactions (≥3%) were constipation (4.5%) and fatigue (4.5%).
- In patients with advanced or recurrent dMMR endometrial cancer in the DUO-E study receiving IMFINZI in combination
with carboplatin and paclitaxel followed by IMFINZI as a single agent (n=44), permanent discontinuation of IMFINZI due
to adverse reactions occurred in 11% of patients. Serious adverse reactions occurred in 30% of patients who received
IMFINZI with carboplatin and paclitaxel; the most common serious adverse reactions (≥4%) were constipation (4.5%) and
rash (4.5%).
Muscle-Invasive Bladder Cancer (MIBC)
- In patients with MIBC, the most common adverse reactions, including laboratory abnormalities, in the overall study (occurring in ≥20% of patients) were decreased hemoglobin, decreased neutrophils, increased blood creatinine, decreased sodium, nausea, increased ALT, decreased calcium, decreased platelets, fatigue, increased potassium, decreased lymphocytes, increased AST, constipation, decreased magnesium, decreased appetite, increased alkaline phosphate, rash, pyrexia, diarrhea, vomiting and abdominal pain.
- In patients with MIBC in the neoadjuvant phase of the NIAGARA study receiving IMFINZI in combination with gemcitabine and cisplatin (n=530), permanent discontinuation of IMFINZI due to an adverse reaction occurred in 9% of patients. Serious adverse reactions occurred in 24% of patients; the most frequent (≥1%) serious adverse reactions were pulmonary embolism (1.9%), febrile neutropenia (1.5%), acute kidney injury (1.3%), thrombocytopenia (1.3%), urinary tract infection (1.3%), and pneumonia (1.3%). Fatal adverse reactions occurred in 1.1% of patients including sepsis, myocardial infarction, and pulmonary embolism (0.2% each). One fatal adverse reaction of pneumonia was reported in 1 (0.2%) patient in the post-surgery phase before adjuvant treatment started. Of the 530 patients in the IMFINZI treatment arm and 526 patients in the chemotherapy treatment arm who received neoadjuvant treatment, 1 (0.2%) patient in each treatment arm did not receive surgery due to adverse reactions. The adverse reaction that led to cancellation of surgery in the IMFINZI treatment arm was interstitial lung disease.
- In patients with MIBC in the adjuvant phase of the NIAGARA study receiving IMFINZI as a single agent (n=383), permanent discontinuation of adjuvant IMFINZI due to an adverse reaction occurred in 5% of patients. Serious adverse reactions occurred in 26% of patients. The most frequent serious adverse reactions (occurring in ≥1% of patients) were urinary tract infection (7%), acute kidney injury (3.7%), hydronephrosis (2.1%), pyelonephritis (2.1%), urosepsis (1.8%) and sepsis (1.6%). Fatal adverse reactions occurred in 1.8% of patients, including COVID-19, severe acute respiratory syndrome, cardiopulmonary failure, gastrointestinal hemorrhage, and chronic hepatic failure (0.3% each).
Resectable Gastric Cancer/Gastroesophageal Junction Adenocarcinoma (GC/GEJC)
- In patients with resectable GC/GEJC, the most common adverse reactions in the overall study (occurring in ≥20% of patients) were diarrhea, nausea, peripheral neuropathy, fatigue, alopecia, decreased appetite, rash, abdominal pain, vomiting, musculoskeletal pain, pyrexia, and stomatitis.
- In patients with resectable GC/GEJC in the neoadjuvant phase of the MATTERHORN study receiving IMFINZI in combination with FLOT chemotherapy (n=475), permanent discontinuation of IMFINZI due to an adverse reaction occurred in 2.5% of patients. Serious adverse reactions occurred in 21% of patients; the most frequent (≥2%) serious adverse reaction was diarrhea (2.5%). Deaths occurred in 1.9% of patients; deaths ≥2 patients included septic shock (0.6%) and acute coronary syndrome (0.4%). Of the 475 patients in the IMFINZI + FLOT chemotherapy treatment arm and 469 patients in the placebo + FLOT chemotherapy treatment arm who received neoadjuvant treatment, 0.6% and 0.4% of patients, respectively, did not receive surgery due to adverse reactions, and 2.3% and 2.6% of patients, respectively, had a delay in surgery due to ARs.
- In patients with resectable GC/GEJC in the adjuvant phase of the MATTERHORN study receiving IMFINZI in combination with FLOT chemotherapy (n=365), permanent discontinuation of IMFINZI due to an adverse reaction occurred in 7% of patients. Serious adverse reactions occurred in 29% of patients; the most frequent (≥2%) serious adverse reaction was pneumonia (2.5%). Deaths occurred in 2.2% of patients; deaths ≥2 patients included gastrointestinal perforation (0.5%) and COVID-19 (0.5%).
- In patients with resectable GC/GEJC in the adjuvant phase of the MATTERHORN study receiving IMFINZI alone (n=345), permanent discontinuation of IMFINZI due to an adverse reaction occurred in 6% of patients. Serious adverse reactions occurred in 14% of patients. Deaths occurred in 1.7% of patients; deaths ≥2 patients included gastrointestinal perforation (0.6%) and COVID-19 (0.6%).
The safety and effectiveness of IMFINZI and IMJUDO have not been established in pediatric patients.
Please see Full Prescribing Information including Medication Guide for IMFINZI and IMJUDO.
You may report side effects
related to AstraZeneca products  .
.