- FOR US HEALTHCARE PROFESSIONALS
- FOR PATIENTS & CAREGIVERS»
- Important Safety Information
- Medical Resources
In unresectable Stage III NSCLC following cCRT
2-YEAR PRIMARY OVERALL SURVIVAL ANALYSIS
(25.2 MONTHS
MEDIAN FOLLOW-UP)1*:
NR
mOS with the PACIFIC Regimen
28.7
months mOS with placebo following cCRT
HR=0.68 (95% CI, 0.53-0.87; P=0.0025)
5-YEAR OVERALL SURVIVAL UPDATE3
The post-hoc 5-year OS analysis was conducted at ~5 years after the last patient was randomized, and was not powered to show statistical significance.3
*The primary 2-year OS analysis was conducted after 299 deaths for 42% maturity (61% of targeted events) with a median follow-up of 25.2 months. Reduction in the risk of death vs placebo was 32% (95% CI, 0.53-0.87) with a log-rank test stratified by sex, age, and smoking history. Median OS was NR with IMFINZI (95% CI, 34.7-NR) vs 28.7 months with placebo (95% CI, 22.9-NR).1,2
†Median OS was 47.5 months with IMFINZI (95% CI, 38.1-52.9) vs 29.1 months with placebo (95% CI, 22.1-35.1). Reduction in the risk of death vs placebo was 28% (HR=0.72; 95% CI, 0.59-0.89) with a log-rank test stratified by sex, age, and smoking history. OS rates with IMFINZI vs placebo were: 83% (95% CI, 79.4-86.2) vs 75% (95% CI, 68.5-79.7) at 12 months, 66% (95% CI, 61.8-70.4) vs 55% (95% CI, 48.6-61.4) at 24 months, 57% (95% CI, 52.0-61.1) vs 44% (95% CI, 37.1-49.9) at 36 months, 50% (95% CI, 45.0-54.2) vs 36% (95% CI, 30.1-42.6) at 48 months, and 43% (95% CI, 38.2-47.4) vs 33% (95% CI, 27.3-39.6) at 60 months.3
cCRT=concurrent chemoradiotherapy; CI=confidence interval; HR=hazard ratio; mOS=median overall survival; NR=not reached; NSCLC=non-small cell lung cancer; OS=overall survival.
In unresectable Stage III NSCLC following cCRT
1-YEAR PRIMARY PFS ANALYSIS
(14.5 MONTHS MEDIAN FOLLOW-UP)1,4*:
16.8
months mPFS with the PACIFIC Regimen
5.6
months mPFS with placebo following cCRT
HR=0.52 (95% CI, 0.42-0.65; P<0.0001)
5-YEAR PROGRESSION-FREE SURVIVAL UPDATE3
The post-hoc 5-year PFS analysis was conducted at ~5 years after the last patient was randomized, and was not powered to show statistical significance.3
In the 5-year post-hoc analysis, IMFINZI demonstrated 36.5 months (95% CI, 28.1-45.2) median TTDM and placebo 17.7 months (95% CI, 12.7-22.1) median TTDM (HR=0.59; 95% CI, 0.47-0.74)3‡
*Measured based on RECIST v1.1 criteria by BICR. The primary PFS analysis was conducted after 371 events (81% of targeted 458 events) with a median follow-up of 14.5 months. Reduction in risk of progression or death vs placebo was 48% (HR=0.52; 95% CI, 0.42-0.65) with a log-rank test stratified by sex, age, and smoking history. Median PFS was 16.8 months with IMFINZI (95% CI, 13.0-18.1) vs 5.6 months with placebo (95% CI, 4.6-7.8).1,3,4
†Measured based on RECIST v1.1 criteria by BICR. Reduction in the risk of progression or death vs placebo was 45% (HR=0.55; 95% CI, 0.45-0.68) with a log-rank test stratified by sex, age, and smoking history. PFS rates with IMFINZI vs placebo were: 56% (95% CI, 51.0-60.2) vs 35% (95% CI, 28.3-40.8) at 12 months, 45% (95% CI, 40.1-49.8) vs 25% (95% CI, 19.3-31.2) at 24 months, 40% (95% CI, 34.7-44.7) vs 21% (95% CI, 15.3-26.9) at 36 months, 35% (95% CI, 29.9-40.1) vs 20% (95% CI, 14.4-26.1) at 48 months, and 33% (95% CI, 28.0-38.2) vs 19% (95% CI, 13.6-25.2) at 60 months.3
‡TTDM was measured at the February 13, 2017 and March 22, 2018 DCOs, and is defined as the time from the date of randomization until the first date of distant metastasis or death in the absence of distant metastasis; distant metastasis is defined as any new lesion that is outside of the radiation field using BICR, according to RECIST v1.1 or proven by biopsy.3
§New lesions were assessed by RECIST v1.1 criteria by BICR and measured during the primary 2-year OS analysis, and the presence of 1 or more new lesions was assessed as progression. A lesion identified at a follow-up assessment in an anatomical location that was not scanned at baseline was considered a new lesion. Patients may have had more than 1 new lesion site. “Other” includes lesions in the biliary tract, chest wall, heart, ovary, pancreas, pericardium, retroperitoneum, skin, spleen, uterus, and other (unspecified).2,3
BICR=blinded independent central review; cCRT=concurrent chemoradiotherapy; CI=confidence interval; DCOs=data cutoffs; HR=hazard ratio; ITT=intent to treat; mPFS=median progression-free survival; NSCLC=non-small cell lung cancer; OS=overall survival; PFS=progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumors; TTDM=time to death or distant metastasis.
In unresectable Stage III NSCLC following cCRT
OVERALL SURVIVAL IN PATIENTS WHO COMPLETED IMFINZI (n=190)†

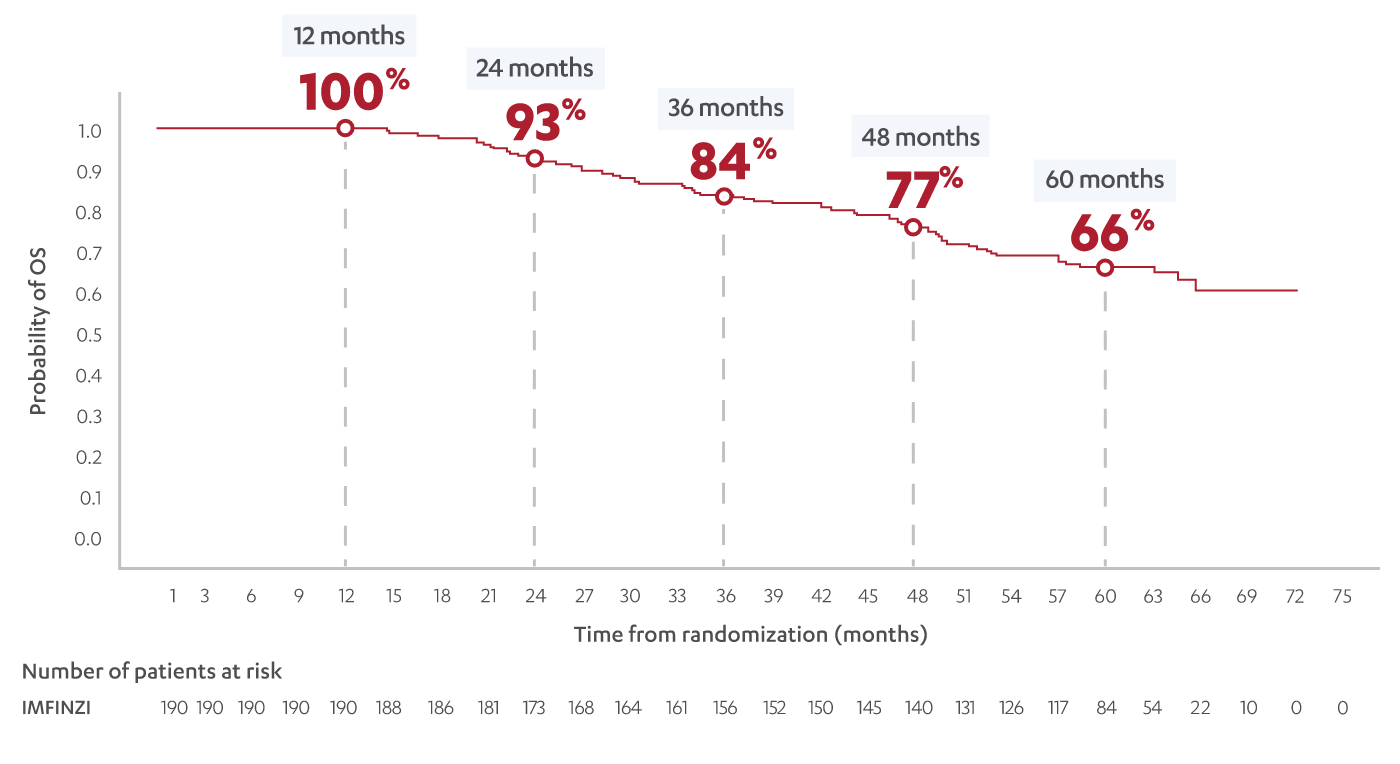
66% of patients who completed treatment* with IMFINZI were alive at 5 years5
IMFINZI PATIENTS
| Baseline characteristics | Completed treatment* n=190 |
ITT‡ n=476 |
|
|---|---|---|---|
| Age | Median (min, max) | 63.0 (36, 83) | 64.0 (31, 84) |
| Gender | Male | 71% | 70% |
| WHO performance status | 0 | 54% | 49% |
| 1 | 46% | 50% | |
| Histology | Squamous | 40% | 47% |
| Nonsquamous | 60% | 53% | |
| Staging by AJCC 7th edition§ | IIIA | 52% | 53% |
| IIIB | 46% | 45% | |
| Best response to previous chemoradiotherapy | CR | 3% | 2% |
| PR | 53% | 49% | |
| SD | 42% | 47% | |
| EGFR or ALK aberration statusII | Negative | 71% | 66% |
| Positive | 5% | 6% | |
| Unknown | 24% | 27% | |
*Completers defined as patients who received 20 to 26 doses once every 2 weeks and had no investigator-assessed disease progression before the end of therapy.5
†Post-hoc OS analysis measured from randomization to IMFINZI for patients whose treatment duration was ≥48 weeks and received ≥20 doses of IMFINZI and who did not progress while on therapy.5
‡~5 years after the last patient was randomized.2,4,5
§According to the AJCC Cancer Staging Manual, 8th edition of the TNM classification for lung cancer derived from databases for the International Association for the Study of Lung Cancer; the study included patients who are now classified as Stage IIIC.2,4,6,7
||Assessment of archived tumor samples obtained before cCRT.4
AJCC=American Joint Committee on Cancer; ALK=anaplastic lymphoma kinase; cCRT=concurrent chemoradiotherapy; CR=complete response; EGFR=epidermal growth factor receptor; ITT=intent to treat; NSCLC=non-small cell lung cancer; OS=overall survival; PFS=progression-free survival; PR=partial response; Q2W=every 2 weeks; SD=stable disease; TNM=tumor, node, metastasis; WHO=World Health Organization.

NCCN CATEGORY 1
Durvalumab (IMFINZI®) is the only NCCN Category 1 post-cCRT consolidation immunotherapy option for unresectable Stage III NSCLC with either a Q2W or Q4W dosing option8*†
*Durvalumab (IMFINZI®) is recommended (Category 1) as a consolidation immunotherapy option for patients with unresectable Stage III NSCLC and performance status 0 to 1 and no disease progression after definitive concurrent CRT (except tumors that are positive for EGFR exon 19 deletion or exon 21 L858R mutations).8 Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer V.8.2025. © 2025 National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. The NCCN Guidelines are a work in progress that may be refined as often as new significant data becomes available.
†NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
‡Based on the modeling of pharmacokinetic data and exposure relationships for safety in patients weighing >30 kg with NSCLC. The steady state AUC is 6% higher, the Ctrough is 19% lower, and Cmax is 55% higher in those who received 1500 mg Q4W compared with those who received 10 mg/kg Q2W.1
ALK=anaplastic lymphoma kinase; AUC=area under the curve; cCRT=concurrent chemoradiotherapy; CRT=chemoradiotherapy; CT=computed tomography; EGFR=epidermal growth factor receptor; FDG=fluorodeoxyglucose; MRI=magnetic resonance imaging; NCCN=National Comprehensive Cancer Network® (NCCN®); NSCLC=non-small cell lung cancer; PD-L1=programmed death-ligand 1; PET=positron emission tomography; PFTs=pulmonary function tests; Q2W=every 2 weeks; Q4W=every 4 weeks.
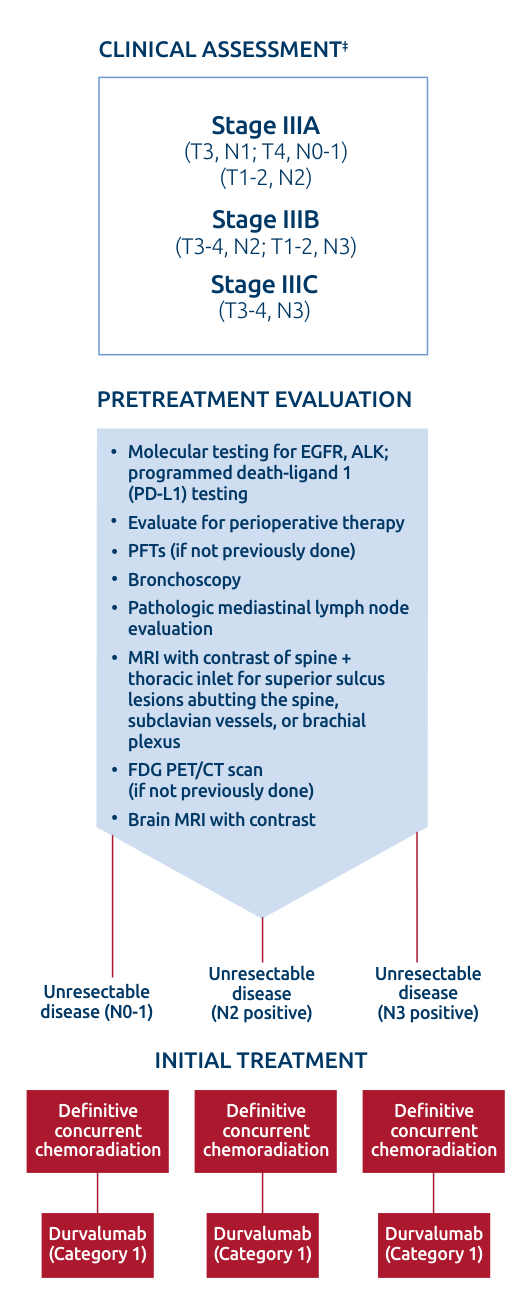
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend evaluation of RT eligibility by a radiation oncologist for all patients with Stage III NSCLC3*
Determination of the appropriateness of radiation therapy should be made by radiation oncologists for all patients with Stage III NSCLC3

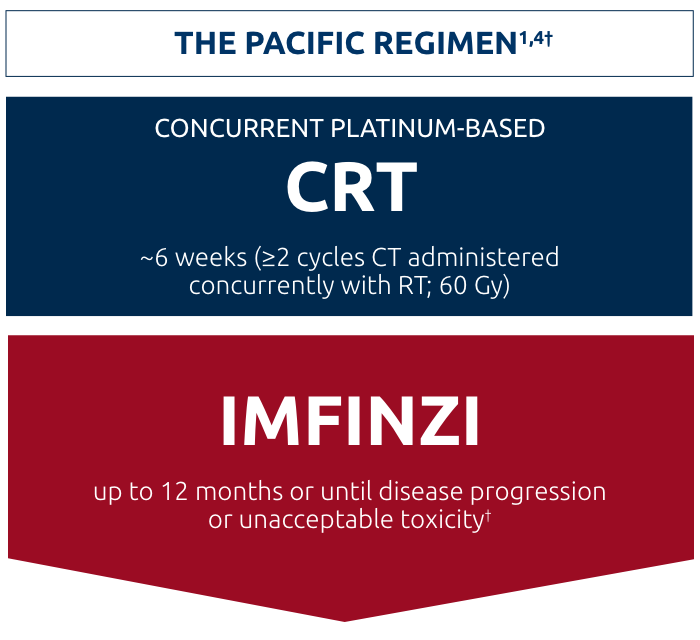
Initiate IMFINZI as soon as clinically possible after the completion of cCRT5
Study design: The PACIFIC study was a large, Phase III, randomized, double-blind, placebo-controlled, international study of 713 patients with unresectable Stage III NSCLC who had not progressed following concurrent, platinum-based CRT. Patients had completed at least 2 cycles of concurrent CRT within 42 days prior to initiation of the study drug and had a WHO performance status of 0 or 1. Randomization at enrollment was stratified according to age, sex, and smoking history. Patients were randomized 2:1 to receive 10 mg/kg of IMFINZI or placebo every 2 weeks for up to 12 months or until unacceptable toxicity or confirmed disease progression. Coprimary endpoints were PFS (measured based on RECIST v1.1 criteria by BICR) and OS. Secondary endpoints included: Percentage of patients alive without disease progression at 12 and 18 months, ORR, DoR, and TTDM.1,5
*NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way.
†In patients with unresectable Stage III NSCLC whose disease has not progressed following concurrent platinum-based chemoradiotherapy.1
‡Refer to Prescribing Information for information on dosage modifications.
§According to RECIST v1.1, patients have stable disease as long as target lesions only grow by <20% and no new lesions develop. Progression is defined as ≥20% increase in the sum of diameters of target lesions, taking as reference the smallest sum on study or the appearance of 1 or more new lesions.6
||The preferred method of assessment in PACIFIC for all lesions was computed tomography. Non-target lesions were also assessed by: MRI, clinical examination, or x-ray/chest x-ray. New lesions were also assessed by: MRI, clinical examination, x-ray/chest x-ray, ultrasound, and bone scan.5
¶Initially, patients with Grade 1 CRT-induced pneumonitis were excluded from the study.5
ARs=adverse reactions; BICR=blinded independent central review; cCRT=concurrent chemoradiotherapy; CRT=chemoradiotherapy; CT=chemotherapy; DoR=duration of response; Gy=gray; MRI=magnetic resonance imaging; NCCN=National Comprehensive Cancer Network® (NCCN®); NSCLC=non-small cell lung cancer; ORR=objective response rate; OS=overall survival; PFS=progression-free survival; RECIST=Response Evaluation Criteria in Solid Tumors; RT=radiotherapy; TTDM=time to death or distant metastasis; WHO=World Health Organization.


IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
There are no contraindications for
IMFINZI® (durvalumab) or IMJUDO® (tremelimumab-actl).
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based...
There are no contraindications for IMFINZI® (durvalumab) or IMJUDO® (tremelimumab-actl).
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and before each dose. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI and IMJUDO depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI and IMJUDO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle-invasive bladder cancer (MIBC).
IMFINZI in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) as neoadjuvant and adjuvant treatment, followed by single agent IMFINZI, is indicated for the treatment of adult patients with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
IMFINZI and IMJUDO can cause immune-mediated pneumonitis, which may be fatal. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
IMFINZI with IMJUDO and platinum-based chemotherapy can cause immune-mediated colitis, which may be
fatal.
IMFINZI and IMJUDO can cause immune-mediated colitis that is frequently associated with diarrhea.
Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory
immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup
to exclude alternative etiologies.
IMFINZI and IMJUDO can cause immune-mediated hepatitis, which may be fatal.
IMFINZI and IMJUDO can cause immune-mediated nephritis.
IMFINZI and IMJUDO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 and CTLA-4 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.
IMFINZI in combination with IMJUDO can cause immune-mediated pancreatitis. Immune-mediated pancreatitis occurred in 2.3% (9/388) of patients receiving IMFINZI and IMJUDO, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions.
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI and IMJUDO or were reported with the use of other immune-checkpoint inhibitors.
IMFINZI and IMJUDO can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI and IMJUDO based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Based on their mechanism of action and data from animal studies, IMFINZI and IMJUDO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and IMJUDO and advise them to use effective contraception during treatment with IMFINZI and IMJUDO and for 3 months after the last dose of IMFINZI and IMJUDO.
There is no information regarding the presence of IMFINZI and IMJUDO in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI and IMJUDO, advise women not to breastfeed during treatment and for 3 months after the last dose.
The safety and effectiveness of IMFINZI and IMJUDO have not been established in pediatric patients.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle-invasive bladder cancer (MIBC).
IMFINZI in combination with fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) as neoadjuvant and adjuvant treatment, followed by single agent IMFINZI, is indicated for the treatment of adult patients with resectable gastric or gastroesophageal junction adenocarcinoma (GC/GEJC).
Please see Full Prescribing Information including Medication Guide for IMFINZI and IMJUDO.
You may report side effects
related to AstraZeneca products  .
.