- FOR US HEALTHCARE PROFESSIONALS
- FOR PATIENTS & CAREGIVERS»
- Important Safety Information
- Medical Resources
For patients with a body weight of ≥30 kg

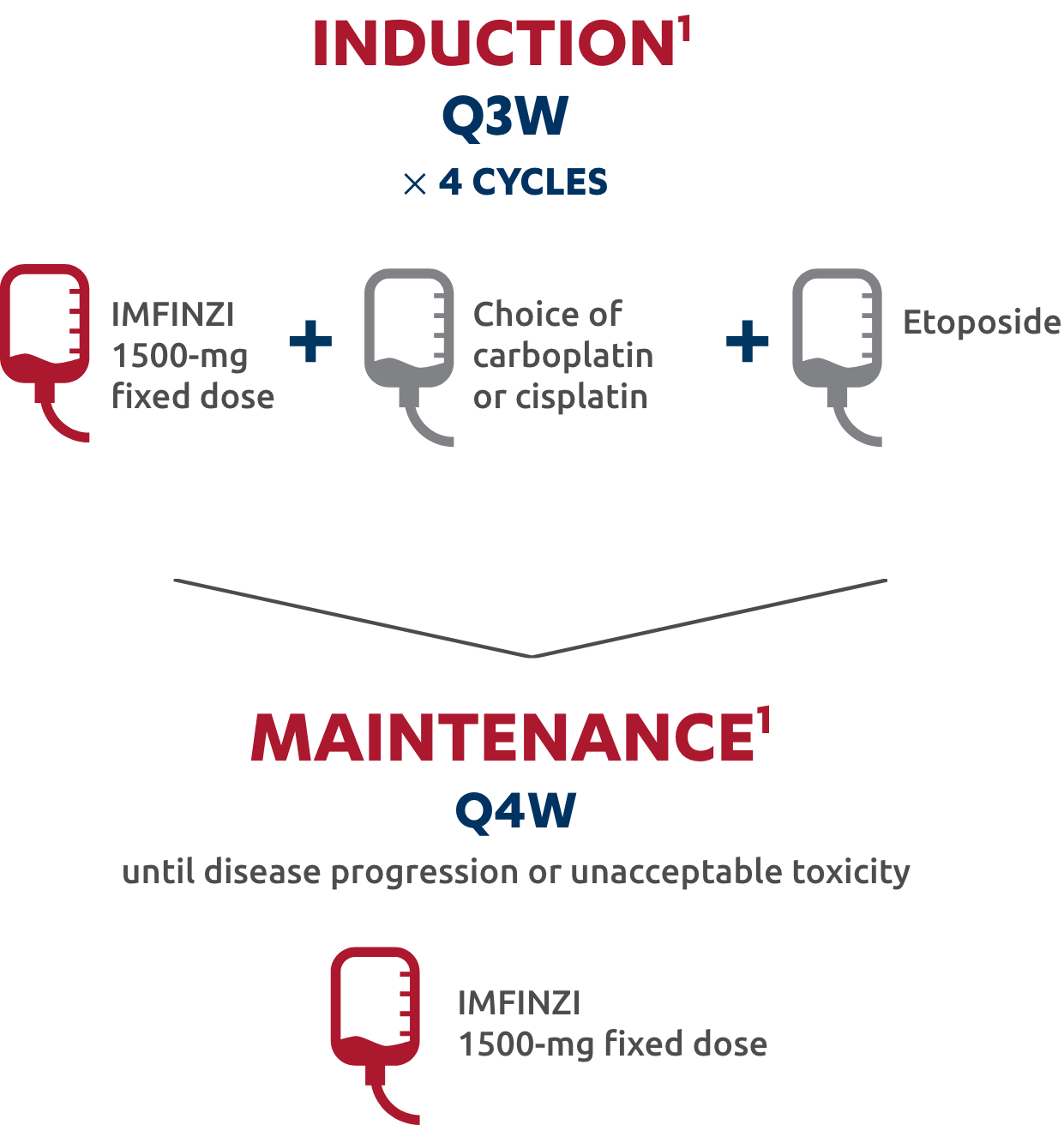
Patients with a body weight of <30 kg must receive weight-based dosing, equivalent to IMFINZI 20 mg/kg in combination with chemotherapy every 3 weeks (21 days) for 4 cycles, followed by 10 mg/kg every 2 weeks as a single agent. EP consists of etoposide 80 mg/m2 to 100 mg/m2 with either carboplatin AUC 5 mg/mL/min or 6 mg/mL/min or cisplatin 75 mg/m2 to 80 mg/m2. For more information, please refer to the Prescribing Information for each treatment.
The only IO combination for first-line ES-SCLC with the option of either carboplatin or cisplatin in chemotherapy1,3
1L=first line; AUC=area under the curve; EP=etoposide and either carboplatin or cisplatin; ES-SCLC=extensive-stage small cell lung cancer; IO=immuno-oncology; Q3W=every 3 weeks; Q4W=every 4 weeks.
Please see Prescribing Information for dosage modification and management specific to adverse reactions.
No dose reduction of IMFINZI is recommended. Withholding or permanently discontinuing IMFINZI due to adverse reactions may be required
SWIPE FOR MORE
SWIPE FOR MORE
Adverse reaction
Immune-mediated adverse reactions
Pneumonitis
Colitis
Intestinal perforation
Hepatitis with no
tumor involvement
of the liver
Hepatitis with
tumor involvement
of the liver‡
Endocrinopathies
Nephritis with
renal dysfunction
Exfoliative
dermatologic
conditions
Myocarditis
Neurological
toxicities
Other adverse reactions
Infusion-related
reactions
Severity*
Grade 2
Grade 3 or 4
Grade 2 or 3
Grade 4
Any grade
AST or ALT increases to >3 and up to 8 × ULN
or
total bilirubin increases to >1.5
and up to 3 × ULN
AST or ALT increases to >8 × ULN
or
total bilirubin increases to >3 × ULN
AST or ALT is >1 and up to 3 × ULN at baseline and increases to >5 and up to 10 × ULN
or
AST or ALT is >3 and up to 5 × ULN at baseline and increases to >8 and up to
10 × ULN
AST or ALT increases to >10 × ULN
or
total bilirubin increases to >3 × ULN
Grade 3 or 4
Grade 2 or 3 increased blood creatinine
Grade 4 increased blood creatinine
Suspected SJS, TEN, or DRESS
Confirmed SJS, TEN, or DRESS
Grade 2, 3, or 4
Grade 2
Grade 3 or 4
Grade 1 or 2
Grade 3 or 4
Treatment modification
Withhold†
Permanently discontinue
Withhold†
Permanently discontinue
Permanently discontinue
Withhold†
Permanently discontinue
Withhold†
Permanently discontinue
Withhold until clinically stable or permanently discontinue depending on severity
Withhold†
Permanently discontinue
Withhold†
Permanently discontinue
Permanently discontinue
Withhold†
Permanently discontinue
Interrupt or slow the rate of infusion
Permanently discontinue
| Adverse reaction | Severity* | Treatment modification |
|---|---|---|
| Immune-mediated adverse reactions | ||
| Pneumonitis | Grade 2 | Withhold† |
| Grade 3 or 4 | Permanently discontinue | |
| Colitis | Grade 2 or 3 | Withhold† |
| Grade 4 | Permanently discontinue | |
| Intestinal perforation | Any grade | Permanently discontinue |
| Hepatitis with no tumor involvement of the liver |
AST or ALT increases to >3 and up to 8 × ULN or total bilirubin increases to >1.5 and up to 3 × ULN |
Withhold† |
| AST or ALT increases to >8 × ULN or total bilirubin increases to >3 × ULN |
Permanently discontinue | |
| Hepatitis with tumor involvement of the liver‡ |
AST or ALT is >1 and up to 3 × ULN at baseline and increases to >5 and up to 10 × ULN
or AST or ALT is >3 and up to 5 × ULN at baseline and increases to >8 and up to 10 × ULN |
Withhold† |
| AST or ALT increases to >10 × ULN or total bilirubin increases to >3 × ULN |
Permanently discontinue | |
| Endocrinopathies | Grade 3 or 4 | Withhold until clinically stable or permanently discontinue depending on severity |
| Nephritis with renal dysfunction |
Grade 2 or 3 increased blood creatinine | Withhold† |
| Grade 4 increased blood creatinine | Permanently discontinue | |
| Exfoliative dermatologic conditions |
Suspected SJS, TEN, or DRESS | Withhold† |
| Confirmed SJS, TEN, or DRESS | Permanently discontinue | |
| Myocarditis | Grade 2, 3, or 4 | Permanently discontinue |
| Neurological toxicities |
Grade 2 | Withhold† |
| Grade 3 or 4 | Permanently discontinue | |
| Other adverse reactions | ||
| Infusion-related reactions |
Grade 1 or 2 | Interrupt or slow the rate of infusion |
| Grade 3 or 4 | Permanently discontinue |
Prescribing Information has additional information for dosage modification and management specific to adverse reactions.
ALT=alanine aminotransferase; AST=aspartate aminotransferase; DRESS=Drug Rash with Eosinophilia and Systemic Symptoms; imARs=immune-mediated adverse reactions; SJS=Stevens-Johnson Syndrome; TEN=toxic epidermal necrolysis; ULN=upper limit of normal.
*Based on National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.
†Resume in patients with complete or partial resolution (Grade 0 to 1) after corticosteroid taper. Permanently discontinue if no complete or partial resolution within 12 weeks of initiating corticosteroids or an inability to reduce corticosteroid dose to 10 mg of prednisone or less per day (or equivalent) within 12 weeks of initiating corticosteroids.
‡If AST and ALT are less than or equal to ULN at baseline in patients with liver involvement, withhold or permanently discontinue IMFINZI based on recommendations for hepatitis with no liver involvement.
IMFINZI does not contain a preservative.
Administer infusion solution immediately once prepared. If the infusion solution is not administered immediately and needs to be stored, the time from preparation should not exceed:
Additionally:
IV=intravenous.


IMFINZI is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
There are no contraindications for IMFINZI® (durvalumab) or IMJUDO® (tremelimumab-actl).
IMFINZI is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not
There are no contraindications for IMFINZI® (durvalumab) or IMJUDO® (tremelimumab-actl).
Important immune-mediated adverse reactions listed under Warnings and Precautions may not include all possible severe and fatal immune-mediated reactions. Immune-mediated adverse reactions, which may be severe or fatal, can occur in any organ system or tissue. Immune-mediated adverse reactions can occur at any time after starting treatment or after discontinuation. Monitor patients closely for symptoms and signs that may be clinical manifestations of underlying immune-mediated adverse reactions. Evaluate clinical chemistries including liver enzymes, creatinine, adrenocorticotropic hormone (ACTH) level, and thyroid function at baseline and before each dose. In cases of suspected immune-mediated adverse reactions, initiate appropriate workup to exclude alternative etiologies, including infection. Institute medical management promptly, including specialty consultation as appropriate. Withhold or permanently discontinue IMFINZI and IMJUDO depending on severity. See USPI Dosing and Administration for specific details. In general, if IMFINZI and IMJUDO requires interruption or discontinuation, administer systemic corticosteroid therapy (1 mg to 2 mg/kg/day prednisone or equivalent) until improvement to Grade 1 or less. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. Consider administration of other systemic immunosuppressants in patients whose immune-mediated adverse reactions are not controlled with corticosteroid therapy.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single-agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle-invasive bladder cancer (MIBC).
IMFINZI and IMJUDO can cause immune-mediated pneumonitis, which may be fatal. The incidence of pneumonitis is higher in patients who have received prior thoracic radiation.
IMFINZI with IMJUDO and platinum-based chemotherapy can cause immune-mediated colitis, which may be
fatal.
IMFINZI and IMJUDO can cause immune-mediated colitis that is frequently associated with diarrhea.
Cytomegalovirus (CMV) infection/reactivation has been reported in patients with corticosteroid-refractory
immune-mediated colitis. In cases of corticosteroid-refractory colitis, consider repeating infectious workup
to exclude alternative etiologies.
IMFINZI and IMJUDO can cause immune-mediated hepatitis, which may be fatal.
IMFINZI and IMJUDO can cause immune-mediated nephritis.
IMFINZI and IMJUDO can cause immune-mediated rash or dermatitis. Exfoliative dermatitis, including Stevens-Johnson Syndrome (SJS), drug rash with eosinophilia and systemic symptoms (DRESS), and toxic epidermal necrolysis (TEN), has occurred with PD-1/L-1 and CTLA-4 blocking antibodies. Topical emollients and/or topical corticosteroids may be adequate to treat mild to moderate non-exfoliative rashes.
IMFINZI in combination with IMJUDO can cause immune-mediated pancreatitis. Immune-mediated pancreatitis occurred in 2.3% (9/388) of patients receiving IMFINZI and IMJUDO, including Grade 4 (0.3%) and Grade 3 (1.5%) adverse reactions.
The following clinically significant, immune-mediated adverse reactions occurred at an incidence of less than 1% each in patients who received IMFINZI and IMJUDO or were reported with the use of other immune-checkpoint inhibitors.
IMFINZI and IMJUDO can cause severe or life-threatening infusion-related reactions. Monitor for signs and symptoms of infusion-related reactions. Interrupt, slow the rate of, or permanently discontinue IMFINZI and IMJUDO based on the severity. See USPI Dosing and Administration for specific details. For Grade 1 or 2 infusion-related reactions, consider using pre-medications with subsequent doses.
Fatal and other serious complications can occur in patients who receive allogeneic hematopoietic stem cell transplantation (HSCT) before or after being treated with a PD-1/L-1 blocking antibody. Transplant-related complications include hyperacute graft-versus-host disease (GVHD), acute GVHD, chronic GVHD, hepatic veno-occlusive disease (VOD) after reduced intensity conditioning, and steroid-requiring febrile syndrome (without an identified infectious cause). These complications may occur despite intervening therapy between PD-1/L-1 blockade and allogeneic HSCT. Follow patients closely for evidence of transplant-related complications and intervene promptly. Consider the benefit versus risks of treatment with a PD-1/L-1 blocking antibody prior to or after an allogeneic HSCT.
Based on their mechanism of action and data from animal studies, IMFINZI and IMJUDO can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. In females of reproductive potential, verify pregnancy status prior to initiating IMFINZI and IMJUDO and advise them to use effective contraception during treatment with IMFINZI and IMJUDO and for 3 months after the last dose of IMFINZI and IMJUDO.
There is no information regarding the presence of IMFINZI and IMJUDO in human milk; however, because of the potential for serious adverse reactions in breastfed infants from IMFINZI and IMJUDO, advise women not to breastfeed during treatment and for 3 months after the last dose.
The safety and effectiveness of IMFINZI and IMJUDO have not been established in pediatric patients.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with unresectable Stage III non-small cell lung cancer (NSCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI in combination with platinum-containing chemotherapy as neoadjuvant treatment, followed by IMFINZI continued as a single agent as adjuvant treatment after surgery, is indicated for the treatment of adult patients with resectable (tumors ≥4 cm and/or node positive) NSCLC and no known epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) rearrangements.
IMFINZI, in combination with IMJUDO and platinum-based chemotherapy, is indicated for the treatment of adult patients with metastatic NSCLC with no sensitizing EGFR mutations or ALK genomic tumor aberrations.
IMFINZI, as a single agent, is indicated for the treatment of adult patients with limited-stage small cell lung cancer (LS-SCLC) whose disease has not progressed following concurrent platinum-based chemotherapy and radiation therapy (cCRT).
IMFINZI, in combination with etoposide and either carboplatin or cisplatin, is indicated for the first-line treatment of adult patients with extensive-stage small cell lung cancer (ES-SCLC).
IMFINZI, in combination with gemcitabine and cisplatin, is indicated for the treatment of adult patients with locally advanced or metastatic biliary tract cancer (BTC).
IMFINZI in combination with IMJUDO is indicated for the treatment of adult patients with unresectable hepatocellular carcinoma (uHCC).
IMFINZI in combination with carboplatin and paclitaxel followed by IMFINZI as a single agent is indicated for the treatment of adult patients with primary advanced or recurrent endometrial cancer that is mismatch repair deficient (dMMR) as determined by an FDA-approved test.
IMFINZI in combination with gemcitabine and cisplatin as neoadjuvant treatment, followed by single-agent IMFINZI as adjuvant treatment following radical cystectomy, is indicated for the treatment of adult patients with muscle-invasive bladder cancer (MIBC).
Please see Full Prescribing Information including Medication Guide for IMFINZI and IMJUDO.
You may report side effects
related to AstraZeneca products  .
.